Experiment VI, Leoben, 20-21 October 2020
This series of experiments amended and continued several earlier ones, such as microfluidics, the viscometer, and emulsion, as well as embarking upon a new one determining the flash point of crude oil.
For the flash point experiment Ernst Logar and Karez Abdulhameed were joined by Michael Hohenberger, assistant at the Chair of Thermal Processing in the Department of Environmental and Energy Process Engineering. The flash point is the lowest temperature at which a flammable substance, in our case crude oil, ignites. For this experiment a Pensky-Martens flash point tester was used. Crude oil was poured into the tester cup and slowly heated, with an ignition flame introduced every 5 to 10 degrees Celsius. The final determined flash point of the sample was at 37.7 degrees Celsius.
Next, Logar and Abdulhameed revisited the microfluidics experiment. This time crude oil was injected into the chip first, then honey. The honey used was much denser than the crude oil, thus displacing the oil quickly. The crude oil was pushed from most of the channels and replaced with the honey. Oil recovery methods aim for similar results, and the properties of the commercial fluids used to enhance oil recovery would be very close to those of the honey.
The rest of the first day of this cycle of experiments was occupied with density measure. Crude oil was injected into a density device, the DMA 35 Anton Paar. The density of the oil sample, provided by the oil and gas company RAG Austria AG, was 0.8816 g/cc. This classifies the oil in the medium class of crude oil with an API of 29.
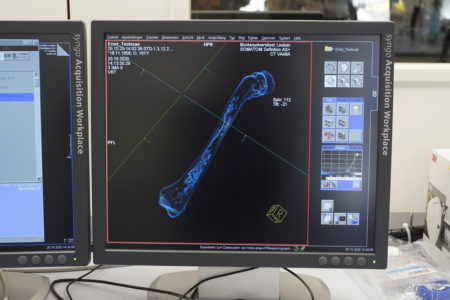
The next afternoon, this time together with Pit Arnold, Logar and Abdulhameed also conducted a short experiment scanning a chicken bone using a CT scan in order to analyse and determine its pore structure. The CT scan image displayed the different structures and contrasts of the bone, but a scanner with higher resolution would be necessary for detailed results.
Afterwards Logar and Abdulhameed revisited the Falling Ball Viscometer experiment. Having filled the falling tube of the viscometer with crude oil, a ball is inserted, the tube closed, and the compartment turned around. Then the falling of the ball in the oil is timed. Based on the time the viscosity of the fluid can be calculated. Gradually increasing oil viscosity in the reservoirs can lead to significant declines in production and different recovery methods are required.
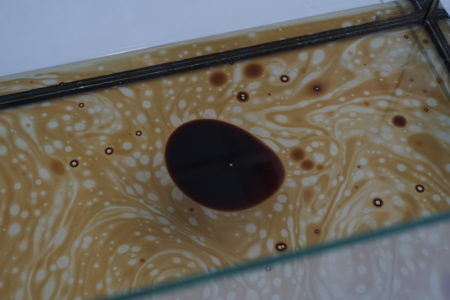
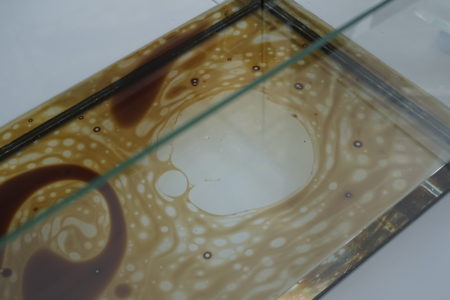
In the final experiment, emulsion was studied again, by filling an aquarium with water, and injecting first crude oil, and then soap. Injecting crude oil formed continuous patterns, while adding the soap pushed the oil away from the centre. In emulsion two or more miscible fluids, such as oil and water, are mixed. Due to their different polarities, the liquids create streams or droplets within or above the other fluid. Introducing a surfactant, in this case the soap, allows the liquids to dissolve by lowering these surface tensions or interfacial tension.
Leoben I experiments I microfluidics I emulsion I flash point I honey I density I bone I CT scan I Falling Ball Viscometer